
Introduction
Bioprinting human tissues and organs that include biomimetic vascular networks is becoming increasingly essential in the field of regenerative medicine. The major challenge lies in creating perfusable channels within cellular and acellular matrices that mirror the complex structures of native blood vessels. A recent study by Stankey et al., published in Advanced Materials, introduces a novel technique called coaxial sacrificial writing into functional tissues (co-SWIFT). This method aims to generate hierarchically branching, multilayered vascular networks within granular hydrogel and densely cellular matrices.
The Co-SWIFT Method
The co-SWIFT technique employs a novel coaxial printhead with an extended core-shell configuration. This configuration facilitates robust core-core and shell-shell interconnections between printed branching vessels. By optimizing core-shell ink combinations, the researchers were able to print biomimetic vessels composed of a smooth muscle cell-laden shell surrounding perfusable lumens within various matrices, including transparent alginate microparticles, sacrificial microparticle-laden collagen, and cardiac spheroids derived from human-induced pluripotent stem cells (hiPSCs).
Creating Biomimetic Vascular Networks
The primary goal of the co-SWIFT method is to create vascular networks that exhibit the complex architectures of native blood vessels. Native vessels are composed of concentrically arranged layers: an endothelial cell layer (intima) regulating barrier function and smooth muscle cells (SMCs) in the medial layer enhancing vessel robustness. Traditional coaxial printing methods, while capable of creating bilayered and trilayered vessels, fall short in replicating the interconnected core-shell structures of natural vascular networks.
To overcome this limitation, the co-SWIFT method combines coaxial embedded and SWIFT bioprinting. The extended core of the coaxial printhead punctures through the shell layer of previously printed features, forming core-core and shell-shell connections. This process enables the creation of branching and reconnecting filamentary architectures within the printed vascular network.
Optimization and Printing Parameters
The researchers optimized the rheological properties of core and shell inks to ensure successful coaxial embedded printing. The granular alginate matrix used in initial experiments allowed direct visualization of the printing process. The optimal core-matrix yield stress (τy) ratio was found to be roughly unity, while the shell-to-matrix τy ratio needed to be around ten times greater. This careful balancing act ensured that the printed core-shell structures maintained their integrity and uniformity, even in complex three-dimensional networks.
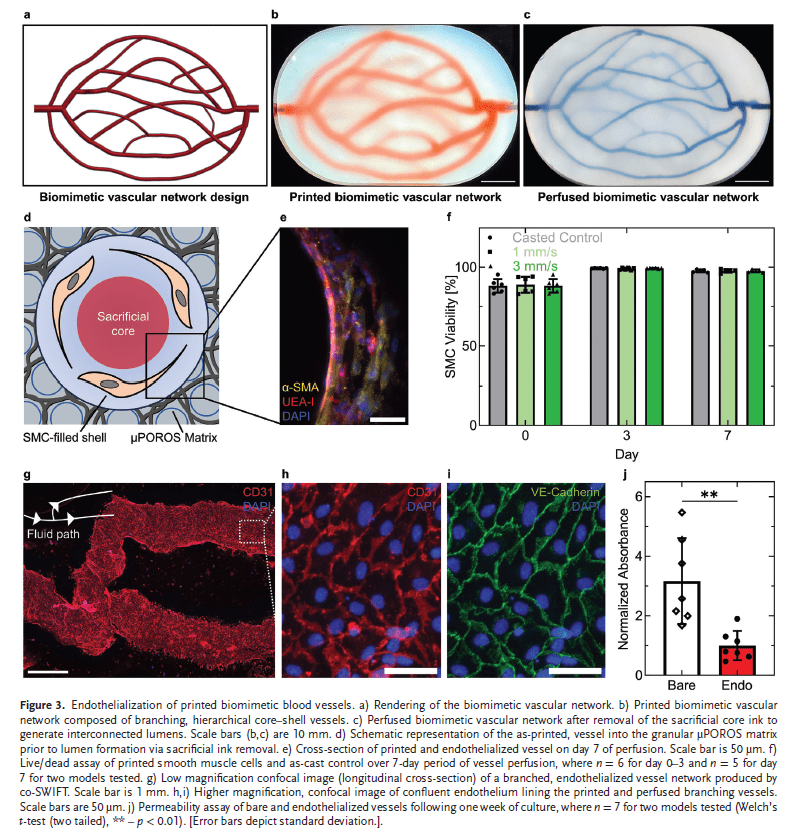
Embedding Vascular Networks in μPOROS Matrices
To demonstrate the versatility of the co-SWIFT method, the researchers embedded biomimetic vascular networks within an extracellular matrix composed of μPOROS collagen. This matrix was created by suspending sacrificial gelatin-chitosan microparticles in a prepolymer collagen solution. The embedded vascular networks were patterned to conform to Murray’s law, ensuring physiological relevance. Upon printing, the sacrificial core was removed, and the luminal surfaces were seeded with endothelial cells, creating functional vessels with good barrier function.
Cardiac Tissue Applications
One of the most exciting applications of the co-SWIFT method is in creating vascularized cardiac tissues. The researchers generated cardiac organ building blocks (cOBBs) from hiPSC-derived cardiomyocytes, which were then embedded with biomimetic vessels. These cardiac tissues, once perfused, exhibited synchronous beating and a cardio-effective drug response in vitro. The co-SWIFT method thus holds promise for creating vascularized human tissues for drug testing, disease modeling, and therapeutic use.
Conclusion
The co-SWIFT method represents an advancement in bioprinting, offering a potentially scalable approach to creating vascularized organ-specific tissues. By enabling the fabrication of hierarchically branching, multilayered vascular networks, this technique opens new avenues for regenerative medicine, particularly in drug testing and disease modeling. The successful integration of smooth muscle cell-laden shells and endothelialized lumens within dense cellular matrices marks a pivotal step towards mimicking the complex architecture of native tissues.
Stankey et al.’s study showcases the potential of co-SWIFT in overcoming the limitations of current bioprinting methods. This innovative technique brings us closer to the goal of creating fully functional, vascularized human tissues and organs, paving the way for future breakthroughs in biomanufacturing and tissue engineering.
References
- Stankey, P. P., Kroll, K. T., Ainscough, A. J., Reynolds, D. S., Elamine, A., Fichtenkort, B. T., Uzel, S. G. M., & Lewis, J. A. (2024). Embedding Biomimetic Vascular Networks via Coaxial Sacrificial Writing into Functional Tissue. Advanced Materials. DOI: 10.1002/adma.202401528
About the Wyss Institute
The Wyss Institute for Biologically Inspired Engineering at Harvard University aims to transform healthcare and the environment by developing innovative bio-inspired technologies. Founded in 2009, the institute draws inspiration from nature’s design principles to create groundbreaking solutions that address some of the world’s most pressing challenges. With a multidisciplinary team of engineers, biologists, chemists, physicists, and clinicians, the Wyss Institute integrates cutting-edge research with real-world applications, spanning areas such as synthetic biology, robotics, regenerative medicine, and nanotechnology. By fostering a collaborative environment and leveraging state-of-the-art facilities, the Wyss Institute accelerates the development of transformative technologies from the laboratory to commercial and clinical use, ultimately aiming to improve human health and environmental sustainability.




Leave a comment