Introduction
Breast reconstruction, augmentation, and revision surgeries have been widely dependent on silicone implants for decades. While these implants have served many patients well, they come with their own set of complications. Issues such as capsular contracture (a painful hardening of scar tissue around the implant), implant rupture, and even rare cases of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) have raised concerns. BellaSeno, a tissue regeneration company is working for a safer and more natural alternative using 3D printing technology.
BellaSeno’s approach revolves around the development of resorbable scaffolds, made from medical-grade polycaprolactone (mPCL). These scaffolds, in combination with a patient’s own fat tissue, offer a new method for breast reconstruction that could reduce risks associated with permanent implants and potentially provide more natural results.
How It Works: The BellaSeno Scaffold
The BellaSeno scaffold is a 3D-printed structure made from mPCL, a biocompatible material that gradually dissolves within the body. These scaffolds are designed to be used in conjunction with fat grafting, where fat is harvested from another part of the patient’s body and used to fill the scaffold. Over time, the scaffold supports the fat as it forms new, natural breast tissue, allowing the body to regenerate the breast in a controlled, predictable way.
What makes this approach unique the biocompatible and resorbable material naturally absorbs in the body over time, leaving behind only the patient’s own tissue. This approach contrasts with traditional silicone implants, which remain in the body indefinitely and carry risks of complications such as implant rupture or the need for replacement surgeries.
The BellaSeno scaffold acts as a protective frame that supports fat grafting, a technique where a patient’s own fat is used to rebuild breast tissue. The scaffold helps the fat grafts maintain their volume and shape, overcoming one of the main challenges of fat grafting alone—significant volume loss. The scaffold shields the fat from pressure exerted by surrounding tissues, allowing for more predictable, long-lasting results.
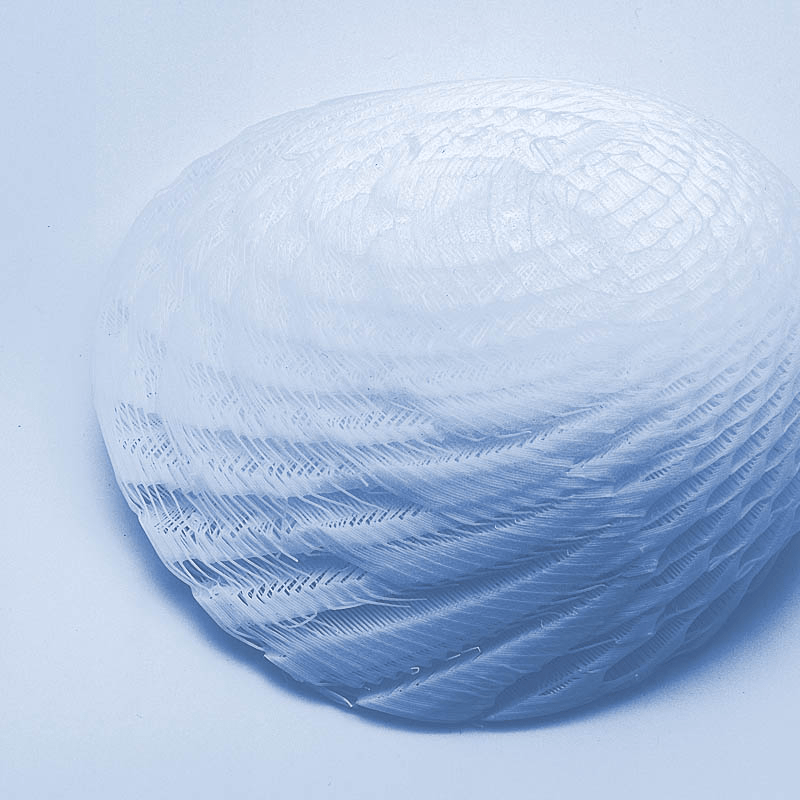
Resorbable breast implants. Image of BellaSeno.
Clinical Trial Results
BellaSeno recently presented preliminary results from their ongoing clinical trial at the 93rd Annual Plastic Surgery Meeting in San Diego. This trial, involving 19 patients undergoing breast implant revision surgery, has already yielded promising outcomes. The trial focuses on evaluating the safety and performance of BellaSeno’s resorbable breast scaffolds in combination with autologous (patient-derived) fat grafting.
So far, 13 patients have completed a 12-month follow-up, with two patients having reached the 24-month mark. Notably, none of the patients have experienced infections, necrosis, or the typical complications seen with silicone implants, such as capsular contracture. More importantly, the results have shown statistically significant improvements in breast volume retention. Patients with the BellaSeno scaffold retained 80% of their breast volume after one year, compared to only 60% for those who underwent traditional fat grafting without the scaffold.
Additionally, patient-reported outcomes demonstrated improvements in overall satisfaction, as well as in areas like sexual and psychosocial well-being. These findings suggest that the scaffold-supported fat grafting process not only improves the physical outcomes but also has a positive impact on patients’ quality of life.
Looking Ahead: Global Expansion and Research
Professor Owen Ung, the Principal Investigator of the study, expressed excitement about the preliminary results and the potential of the technology to improve outcomes for patients globally. He highlighted that the company plans to expand the study into an international multi-center trial, which will further validate the technology and bring it to more patients around the world.
The long-term safety and effectiveness of the mPCL scaffold will continue to be monitored, with final results expected in the coming years. However, the initial findings already indicate that this resorbable scaffold could represent a safer, more natural alternative to traditional breast implants.
About BellaSeno
BellaSeno GmbH was founded in 2015 and is headquartered on the BioCity campus in Leipzig, Germany, with a subsidiary in Brisbane, Australia. The Company is developing novel resorbable soft tissue and bone reconstruction implants made by additive manufacturing (3D-printing) under EU MDR certification. The Company has received substantial financial support from private investors as well as from the Saxony Development Bank (SAB), the European Fund for Regional Development (EFRE), Germany´s Federal Ministry of Education and Research (BMBF) and the Australian government. The Company has been co-funded from tax resources based on the budget adopted by the members of Saxony State Parliament.
Original text found here.


Leave a comment