In tissue engineering, the development of functional vascular networks remains one of the greatest challenges. Vascularization—the formation of blood vessel networks—is essential for delivering nutrients, oxygen, and waste removal in any tissue construct intended for clinical use. A recent study, published in Advanced Healthcare Materials, introduces an approach that leverages programmable bioinks to assort vascular endothelial growth factor (VEGF) presentation, offering control over vascular network formation in engineered tissues.
This work is innovative because it combines spatial patterning and temporal control to guide vascular morphogenesis, mimicking the body’s natural processes more. Let’s dive into how these programmable bioinks work and why this innovation has implications for bioprinting.
The Challenge of Building Vascular Networks
In the body, blood vessels form through tightly regulated processes, guided by spatial and temporal presentation of biochemical signals like VEGF. These signals form gradients that direct endothelial cells to migrate, align, and assemble into hierarchically organized vascular networks.
In traditional bioprinting approaches, VEGF is often delivered in high concentrations and continuously available throughout the construct. While this method ensures VEGF is present, it lacks the precision of natural systems, often leading to excessive, disorganized, or leaky vascular networks. This can undermine the functionality and integration of engineered tissues into the body.
To address this, the Dr. Rouwkema and his team developed a programmable bioink that combines spatial control (where VEGF is located) and temporal control (when VEGF is released), enabling the creation of vascular networks that closely resemble those in the body.
How Programmable Bioinks Work
The researchers designed bioinks functionalized with aptamers—short DNA sequences that can bind VEGF with high specificity. These aptamers act as molecular “locks” that hold VEGF in place until an external trigger is applied.
- Sequestration: VEGF is tightly bound by the aptamers within specific regions of the bioink, ensuring it is unavailable to cells until needed.
- Controlled Release: A complementary DNA sequence (CS) is added to the medium at a chosen time, displacing VEGF from the aptamers. This release mimics natural processes in the body, such as enzymatic breakdown of the extracellular matrix, which regulates growth factor availability during tissue repair.
- Localized Gradients: The released VEGF diffuses into neighboring regions, creating gradients that guide endothelial cells to migrate, align, and form structured vascular networks.
This level of control—delayed, localized, and patterned VEGF release—represents an new interesting way as to how vascular morphogenesis is engineered in bioprinted tissues.
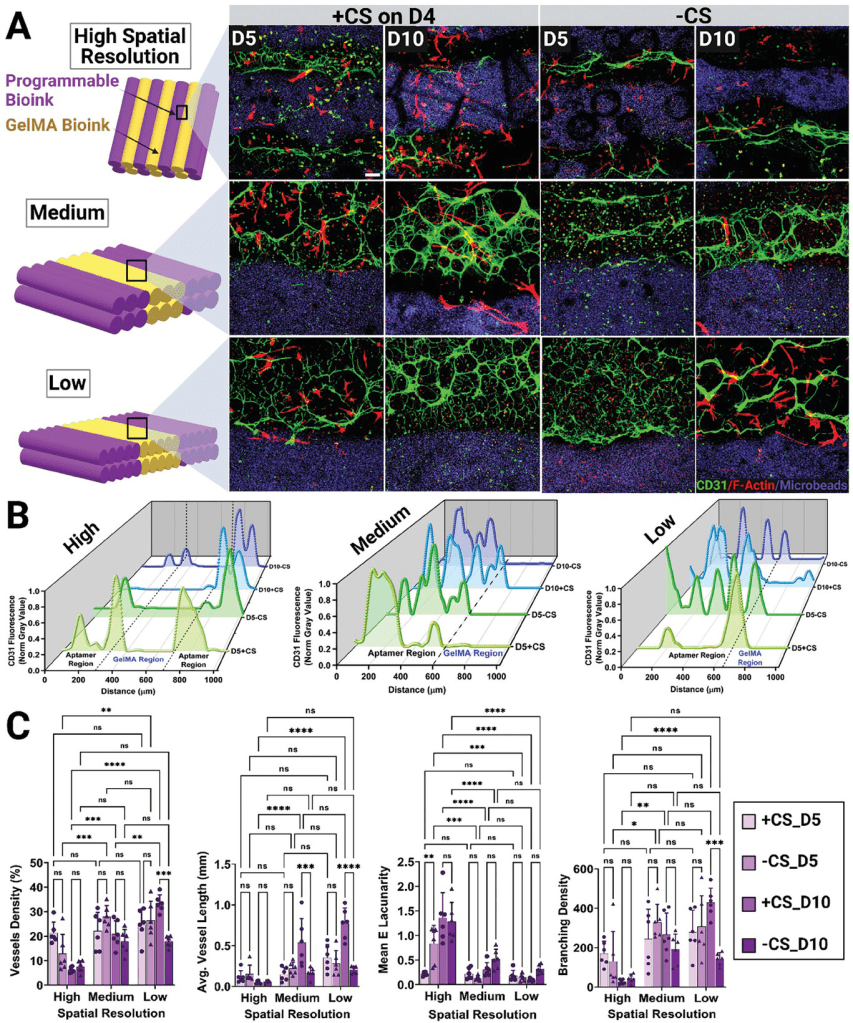
This figure demonstrates how spatial resolution and complementary sequence (CS) triggering influence vascular network formation in bioprinted constructs over time. Low-resolution designs (wider aptamer regions) paired with CS-triggering(+CS) exhibited the most robust and hierarchical vascular networks, with significant improvements in vessel density, branching density, and average vessel length by day 10. High-resolution designs created more localized networks, while low-resolution designs facilitated broader, more interconnected structures, mimicking natural vascular organization. The confocal images highlight the spatial differences in endothelial cell organization and the effectiveness of CS-triggered VEGF release in guiding vascular morphogenesis.
Summary of Key Findings from the Study
- Why Low-Resolution Works Best:
- Better Diffusion: Broad VEGF gradients in low-resolution designs allow VEGF to diffuse farther, influencing larger areas for endothelial cell migration and alignment.
- Enhanced Network Complexity: Supports dense, branched, and elongated vessels across a broader area.
- Mimics Natural Systems: Extended gradients resemble natural VEGF distribution, resulting in more functional and realistic vascular networks.
- Key Results from Low-Resolution Designs with CS:
- Achieved the highest vessel density, most interconnected networks, and longest vessels.
- Ideal for applications requiring extensive vascularization, such as large-scale engineered tissues.
- Improved Vascular Properties:
- Delayed VEGF release via CS-triggering led to:
- 1.35-fold increase in vessel density.
- 1.54-fold increase in branching density.
- 2.19-fold increase in average vessel length compared to controls with no VEGF release.
- Delayed VEGF release via CS-triggering led to:
- Mimicking Natural Processes:
- VEGF release was spatially and temporally controlled to replicate natural growth factor presentation.
- Delayed release enabled endothelial cells to organize and migrate before VEGF activation, improving alignment and network structure.
- Temporal and Spatial Precision:
- High-resolution designs formed sharp VEGF gradients for localized effects, while low-resolution designs created broader gradients for extensive vascularization.
- Avoiding Common Pitfalls:
- The spatiotemporal control of VEGF prevented chaotic or leaky vessel formation, producing stable, hierarchical, and functional vascular networks, overcoming issues with traditional continuous VEGF exposure methods.
This study on programmable bioinks showcases how spatiotemporal control over VEGF presentation can unlock possibilities for vascularized tissue engineering. By mimicking the body’s natural mechanisms, this approach paves the for more functional, clinically relevant tissue constructs.
About the Investigator
Jeroen Rouwkema, Ph.D., is an Associate Professor at the University of Twente and a leading expert in tissue engineering and regenerative medicine. His research focuses on creating vascularized tissues by combining biomaterials science, bioprinting, and computational modeling to mimic natural systems. Dr. Rouwkema’s innovative work on programmable bioinks highlights his commitment to advancing functional tissue engineering, offering transformative solutions for clinical applications.
Find the paper here.




Leave a comment