4D Bioprinting: Not a New Concept, But What Makes This Study Unique?
The concept of 4D bioprinting—creating printed constructs that transform over time—has been around for over a decade. However, recent work, in Advanced Functional Materials out of Andrew Daly’s lab, are pushing the boundaries of this technology by integrating cell biology, computational modeling, and bioprinting techniques. This study stands out for its ability to predict and program how bioprinted tissues morph and mature, leveraging the natural forces generated by cells. But how exactly does it work, and why does it matter?
How Does the Technology Work?
This research demonstrates a novel 4D bioprinting approach that uses cell-generated forces to drive predictable shape-morphing in bioprinted tissues. Here’s a breakdown of the process:
- Bioink Composition: The researchers developed a collagen-based bioink with added hyaluronic acid to ensure high viscosity and support cell growth. This bioink was optimized to allow cell-generated contraction forces to drive tissue transformation.
- Embedded Bioprinting: Constructs were printed into a soft, granular support bath. This bath not only supports the initial 3D shape but also provides mechanical resistance that guides cell and extracellular matrix (ECM) organization as the tissue morphs.
- Shape-Morphing Driven by Cells: The study shows that cells—particularly cardiac fibroblasts and induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs)—generate forces that shrink and reshape the bioprinted tissue. Over time, these forces align the cells and ECM along the tissue’s principal axis, mimicking the anisotropic architecture found in native heart tissue.
- Predictive Computational Modeling: Using a finite element model (FEM), the researchers could simulate and predict how the bioprinted tissues would morph based on factors like bioink stiffness, cell density, and geometry. This computational tool allows for precise programming of tissue shape and structure before printing.
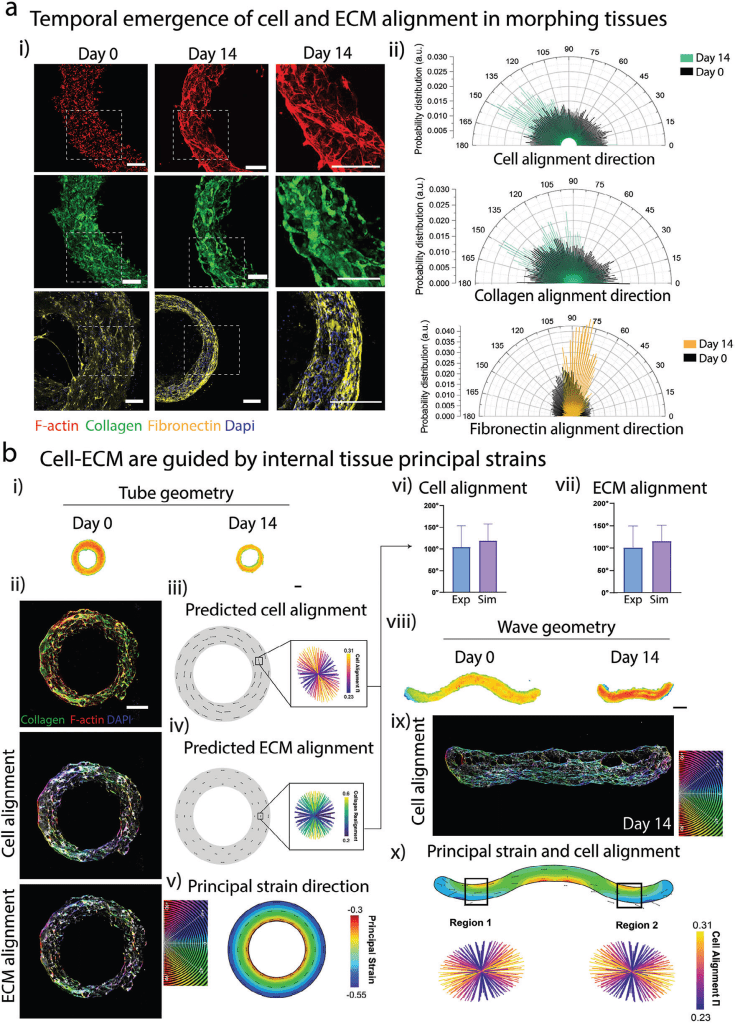
Figure from publicaiton: Modulating 4D shape-morphing via bioink composition and cell phenotype
Why Does This Matter?
1. A Developmentally Inspired Approach
Unlike traditional bioprinting, which aims to recreate the final structure of tissues, this method mimics embryonic organ development. The tissue undergoes dynamic transformations, sculpting itself into a mature and functional form. This paradigm shift could open new possibilities for creating complex organs, like the heart, with intricate internal architectures.
2. Enhanced Maturation of iPSC-Derived Heart Tissues
One of the most impressive results was the structural and functional maturation of iPSC-CMs. In traditional setups, these cells often remain immature, limiting their utility for regenerative medicine. However, in this study, shape-morphing promoted:
- Circumferential alignment of cells and ECM, critical for heart tissue functionality.
- Mature sarcomere development, a hallmark of adult cardiomyocytes.
- Improved contractility, with synchronized and robust beating observed in bioprinted heart tubes.
3. Scalability and Versatility
The platform demonstrated scalability by successfully printing and morphing larger tissue constructs, such as multi-chamber heart models. The use of embedded printing in granular hydrogels also allows for customization of complex geometries.
What Sets This Study Apart?
While 4D bioprinting is not a new concept, the novelty here lies in the integration of predictive modeling, cell-mediated morphogenesis, and endogenous mechanical stimulation. This creates a system where:
- Tissue shape and internal architecture are sculpted dynamically, as opposed to being fixed during printing.
- Maturation is driven by natural processes, reducing reliance on artificial stimulants like bioreactors.
- The approach is modular and programmable, enabling researchers to tailor tissues for specific applications, from vascular grafts to organ-scale constructs.
Challenges and Future Directions
Despite its promise, this method is not without challenges. Key areas for improvement include:
- Incorporating vascular networks for nutrient delivery in thicker tissues.
- Integrating volumetric growth to mimic organ expansion during development.
- Adapting for clinical scalability, ensuring cost-effectiveness and reproducibility.
Conclusion: The Potential Future of Organ Engineering
This study showcases how 4D bioprinting, inspired by natural developmental processes, can influence tissue engineering. By harnessing the power of cell-generated forces and predictive modeling, researchers have taken a step toward creating more functional, mature tissues. This innovative framework sets the stage for a future where bioprinted tissues that are more similar to their natural counterparts.




Leave a comment