A team of bioengineers at Carnegie Mellon University has unveiled a new tissue engineering platform. The study, published in Science Advances, introduces CHIPS (Collagen-based High-resolution Internally Perfusable Scaffolds), a new class of biological, perfusable 3D-printed tissue constructs, and VAPOR, a customizable perfusion bioreactor designed to support them.
What’s the Technology?
The platform uses an improved version of FRESH (Freeform Reversible Embedding of Suspended Hydrogels) bioprinting to directly print native extracellular matrix proteins—especially collagen type I—along with living cells into soft, high-resolution scaffolds that include intricate internal channels for fluid flow. These channels mimic vasculature, enabling nutrient delivery and waste removal in 3D tissues up to centimeter scale.
To support perfusion of the soft CHIPS, the team developed VAPOR, a 3D-printed bioreactor that integrates with CHIPS through barbed connections, enabling dynamic culture and long-term perfusion.
What’s New Here?
- All-biologic fabrication: Unlike conventional organ-on-chip systems made from plastics or synthetic resins, CHIPS are built from ECM components like collagen and fibrin—materials that cells can remodel and integrate with.
- High-resolution perfusable networks: The system achieves internal channels as small as ~100 μm, printed directly within the scaffold, without the need for post-seeding.
- Multi-material, multi-cellular printing: The upgraded printer uses three materials simultaneously to spatially pattern ECM stiffness, bioactive proteins (e.g., VEGF), and distinct cell types—including endothelial and pancreatic cells.
- Functional tissue engineering: The team demonstrated a pancreatic-like CHIPS that responds to glucose stimulation by secreting insulin, as well as endothelial-lined vessels expressing markers like VE-Cadherin and CD31.
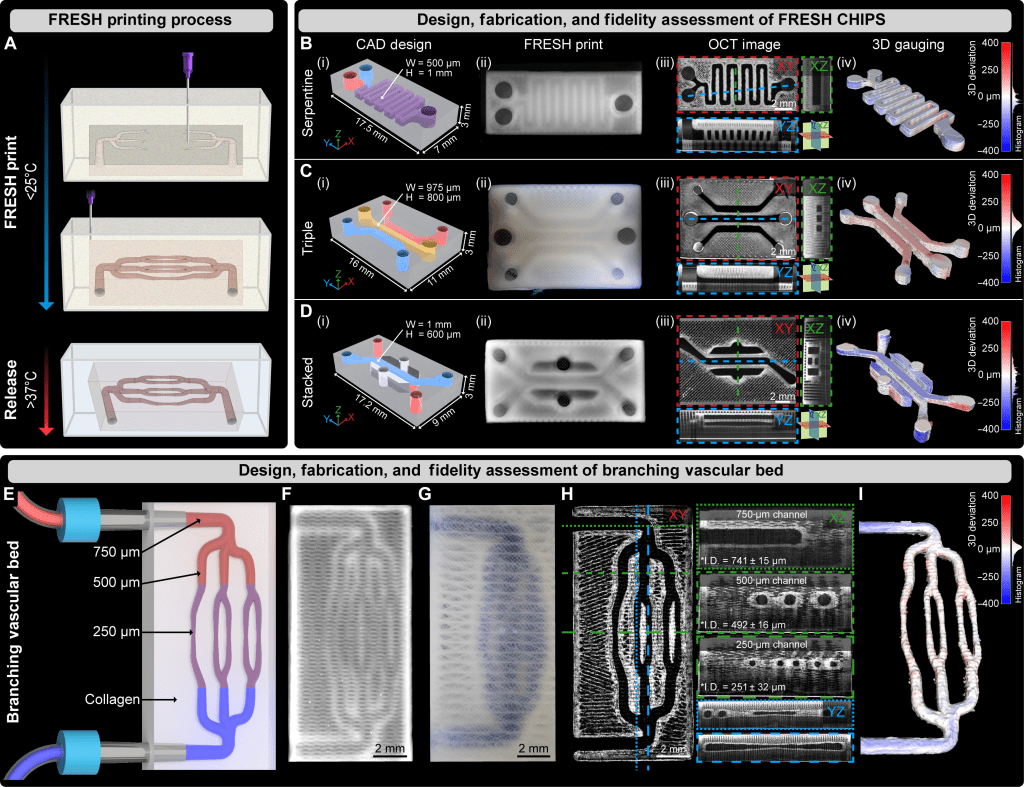
Figure from paper: Fabrication, 3D gauging, and perfusion of FRESH-printed CHIPS.
Why It’s Useful for Products
This platform closes a longstanding gap in tissue engineering: the ability to build biologically relevant, perfusable tissues at functional scales with internal complexity. It removes the dependence on rigid, non-remodelable materials like PDMS and plastic—materials ill-suited for implantation or host integration.
Potential product applications include:
- In vitro disease models and drug testing platforms that more accurately reflect human biology, reducing animal use and clinical trial failures.
- Custom, implantable therapeutic tissues such as engineered vasculature or endocrine tissues for diabetes treatment.
- Pre-vascularized grafts for regenerative medicine, with improved integration and reduced fibrosis risk due to native ECM composition.
Importantly, the system is open-source friendly and adaptable. It can be built using commercial bioprinters or even modified consumer-grade printers, lowering barriers to adoption for labs and startups alike.
As biofabrication moves toward clinical translation, technologies like CHIPS and VAPOR may prove pivotal in enabling scalable, biomimetic tissue production that meets both research and therapeutic needs.
For more information, check out the paper.




Leave a comment