New algorithm offers scalable design pathway for patient-specific blood vessel networks
Addressing a Core Challenge in Biofabricated Organs
Stanford researchers have introduced a computational tool that could remove a major roadblock in organ bioprinting: the creation of functional, patient-specific vascular networks. Their new platform, published in Science on June 12, 2025, enables the rapid design and conversion of complex blood vessel architectures into 3D-printable formats. This development could help bridge the gap between lab-grown tissues and fully implantable organs.
More than 100,000 people in the U.S. remain on organ transplant waiting lists. Biofabrication aims to address this shortfall by engineering organs using patients’ own cells, potentially eliminating issues related to donor shortages and immune rejection. However, scaling up engineered tissues has been limited by the inability to replicate the body’s intricate vascular systems.
Algorithmic Generation of Personalized Vasculature
Led by Professors Alison Marsden and Mark Skylar-Scott, the Stanford team developed an algorithm that generates realistic vascular “trees” at organ scale. The model simulates fluid dynamics and maintains biological constraints like looped systems, branch spacing, and tissue penetration depth. Notably, it can complete designs for human-scale organs—like a heart—in under six hours, versus months using prior techniques.
The vascular tree designs were published through the open-source SimVascular platform, enabling broad academic and commercial access.
Demonstration and Validation
The team demonstrated the platform by printing a simplified vascular model with 500 branches and embedding it into a tissue ring seeded with human kidney cells. When perfused with nutrient solution, the surrounding cells remained viable—indicating early proof of function.
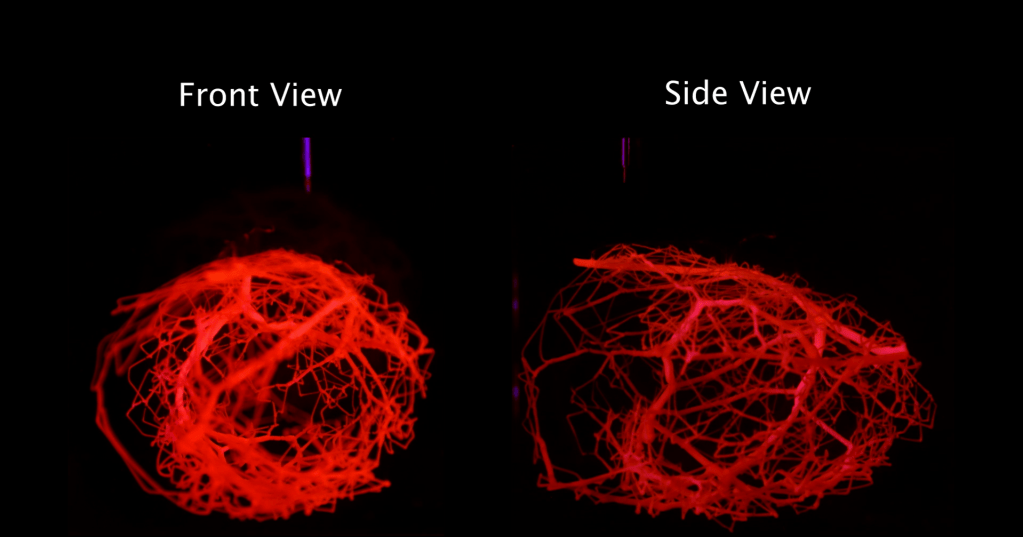
Above is the aforementioned 500-vessel interconnected synthetic vascular network fabricated within a bi-ventricle structure from figure 3 in the paper.
The research marks a significant technical milestone but acknowledges that the printed vessels currently lack critical cell types such as endothelium and smooth muscle. The networks serve as structural channels, not fully functional vasculature.
Limitations and Future Work
The next steps involve integrating biological cells into the printed structures and improving printer speed and resolution. Researchers are also investigating methods to stimulate in vivo microvascularization, especially for vessels too small to print directly.
In parallel, the team is scaling up cell manufacturing to support whole-organ bioprinting, such as the creation of a patient-specific heart.
About the Authors
Alison L. Marsden, Ph.D.
Dr. Alison Marsden is the Douglas M. and Nola Leishman Professor of Cardiovascular Diseases and a Professor of Pediatrics and Bioengineering at Stanford University. With a joint appointment in the Schools of Engineering and Medicine, she leads research at the intersection of computational modeling and regenerative medicine. Marsden’s lab has pioneered patient-specific cardiovascular simulations and vascular design algorithms, culminating in the open-source SimVascular platform. As co-senior author, she directed the development of the high-speed vascular generation algorithm featured in Science, which promises to accelerate scalable, clinically relevant bioprinting of organ vasculature at scale.
Mark A. Skylar‑Scott, Ph.D.
Dr. Mark Skylar-Scott is an Assistant Professor of Bioengineering at Stanford University and a leader in the BASIC (Basic Science and Engineering) Initiative at the Stanford Children’s Heart Center. His research focuses on advancing bioprinting technologies for heart and vascular tissue engineering. As co-senior author, he helped translate the computational vascular models into physical constructs—demonstrating the ability to bioprint complex, perfusable vascular structures. His work bridges engineering, cell biology, and translational medicine, advancing organ-scale regenerative therapies.
For more info go to their paper




Leave a comment