Revalia Bio, a Yale-born startup redefining drug development through real human donor tissue, has secured a landmark ~$30 million U.S. government contract—one of the largest ever awarded to a seed-stage biotechnology company. The announcement marks a turning point not only for Revalia, but for the broader movement away from animal-model drug development and toward human-first biology.
While much of the buzz around the award has focused on the size of the contract, the more important question is: What exactly has Revalia built that justifies this level of government confidence?
Below, we unpack Revalia’s platform, how it works, and what this funding will enable next.
1. What Revalia Bio Has Built: A Human-Tissue Infrastructure for Drug Development
Where most biotech companies develop therapeutics, Revalia has focused on a different layer of the value chain: the biological infrastructure needed to test, validate, and accelerate therapeutics using real human tissue—before any clinical trials begin.
Their core insight: Every human organ donor is a dataset—an opportunity to measure real human biology at unmatched resolution.
Revalia’s platform transforms donated organs into standardized, high-fidelity translational tools that drug developers can use to measure:
- Pharmacology
- Toxicity
- Metabolism
- Tissue-specific responses
- Inter-individual variability
This creates a system they call Human Data Trials™—a preclinical phase that happens between the animal stage and the first-in-human study, using actual functional human tissue rather than predictions about human behavior.
2. How Revalia’s Platform Works
Revalia’s infrastructure integrates:
A. Organ Procurement + Functional Preservation
Using organ-donation networks, Revalia obtains tissues from donors that would otherwise be discarded. They apply preservation technologies that maintain:
- Perfusion
- Viability
- Tissue architecture
- Cellular function
Unlike static samples, these preserved tissues behave dynamically, allowing true pharmacological testing.
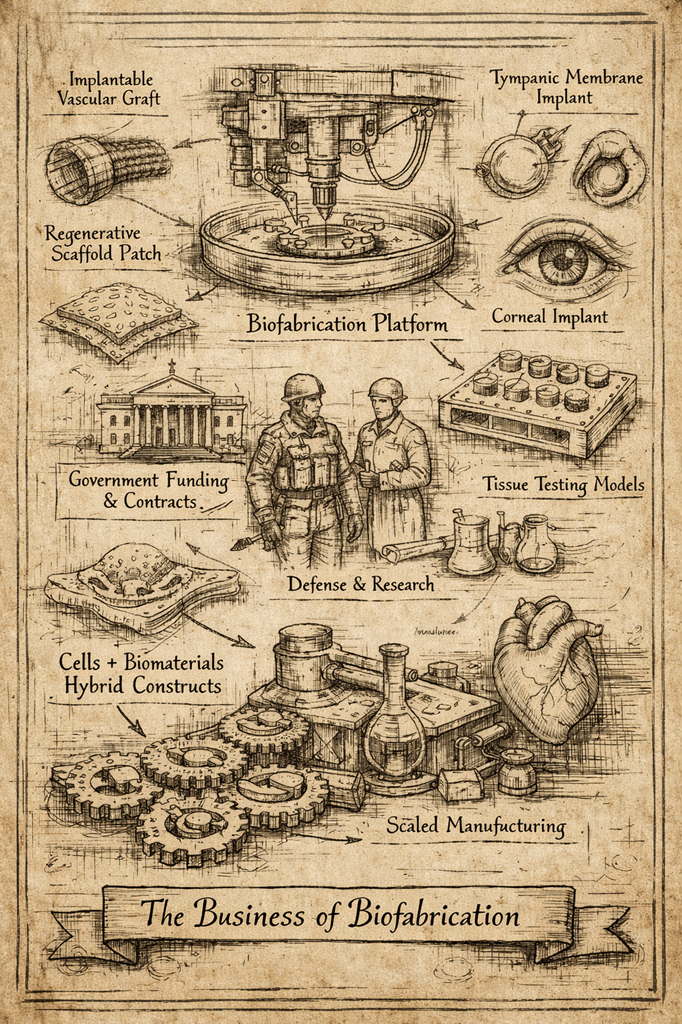
B. Tissue Processing and Standardization
Revalia performs:
- Controlled slicing
- Perfusion chamber integration
- Mechanical and biochemical stabilization
- Precise metadata tagging (donor age, genotype, health history)
This transforms each organ into a standardized experimental substrate, something the industry has lacked for decades.
C. Ex Vivo Drug Response Testing
Drugs are applied to these preserved tissues under controlled conditions, generating high-resolution human-specific data:
- Real-time physiological changes
- Multi-omics response profiles
- Metabolic signatures
- Toxicity thresholds
- Dose responses
This is not simulation and not an organ-on-chip abstraction.
It is actual human tissue responding to actual drug exposure.
D. Computational Integration
Revalia uses analytics and machine learning to correlate:
- How tissues from different donors behave
- How disease-specific tissues respond
- How genetic signatures influence drug susceptibility
This informs early decision-making for pharma and government partners.
3. Why the U.S. Government Awarded Revalia ~$30M
This contract validates a strategic need:
Defense and civilian agencies require predictive human models to accelerate medical countermeasures before crises occur.
Revalia’s human-tissue infrastructure allows agencies to:
- Model human responses to new pathogens, toxins, and therapeutics
- Identify safety signals early
- Reduce dependence on slow and unreliable animal models
- Rapidly evaluate countermeasures in a scalable, data-rich environment
- Generate real human data without exposing human subjects
This is national biodefense infrastructure, built privately, years ahead of its time.
4. What Revalia Will Use the Funding For
The new capital enables Revalia to:
A. Expand Organ-Tissue Processing Capacity
More sites, more donor throughput, more tissue types.
B. Scale Human Data Trials™ for government and pharma programs
Increase the volume and diversity of human tissue testing.
C. Build Advanced Data Systems
Integrate genomics, proteomics, functional tissue responses, and historical donor data into unified predictive models.
D. Establish Long-Term Biological Infrastructure
Revalia aims to become the standardized national repository and testing system for human functional biology—filling a gap animal models cannot solve.
5. Why Revalia Represents a New Category in Biofabrication
While Curi Bio, Emulate, AxoSim, and others build engineered human models, Revalia leapfrogs the industry by using native human tissues directly.
This creates:
- The highest-fidelity biological testbed available
- A system capable of population-level pharmacology
- Data grounded in real human variability (age, genetics, disease history)
- A translational bridge no model can yet replicate
Revalia isn’t a therapeutics company.
It is biomedical infrastructure, built on the world’s most accurate biological material: actual human tissue.
About Revalia Bio
Revalia Bio is a Yale spinout building the world’s first large-scale infrastructure for Human Data Trials™, enabling drug developers to test therapeutics on real human tissues before entering clinical studies. With backing that includes $20M in funding, ~$30M in government contracts, and ~$40M in research grants, Revalia is redefining the translational pipeline by replacing animal models and predictions with real human biology.
Website: revaliabio.com


Leave a comment